Elon Musk’s Neuralink, a company on the cutting edge of neuroscience technology, has encountered a setback with its first brain implant in a human subject. Despite the initial success, the device experienced issues with its functionality, raising questions about the future of brain-computer interfaces (BCIs).
The Dawn of a New Era in Neuroscience
Neuralink, founded by visionary entrepreneur Elon Musk, has been developing a state-of-the-art BCI known as the Link. This device, designed to potentially assist paralyzed individuals in controlling external technology through thought alone, is equipped with 1,024 electrodes distributed across 64 ultra-thin threads. This complex system promises a new level of interaction between the human brain and machines.
In a landmark moment for the company, Neuralink implanted the Link in 29-year-old Noland Arbaugh, a participant in a study aimed at testing the device’s safety. The procedure, which took place in January, was initially deemed a success by Neuralink, as evidenced by a live demonstration in March where Arbaugh skillfully used the BCI.
Elon Musk’s Neuralink chip suffers unexpected setback in first in-human brain implant.
The chip began to detach for the skull reducing its data capture ability. pic.twitter.com/awunwwmL1I
— DramaAlert (@DramaAlert) May 9, 2024
Encountering Unforeseen Challenges
However, the triumph was short-lived. Neuralink disclosed in a recent blog post that several threads had retracted from Arbaugh’s brain tissue, leading to a decrease in the number of effective electrodes. This issue compromised the Link’s ability to accurately and swiftly record neural signals.
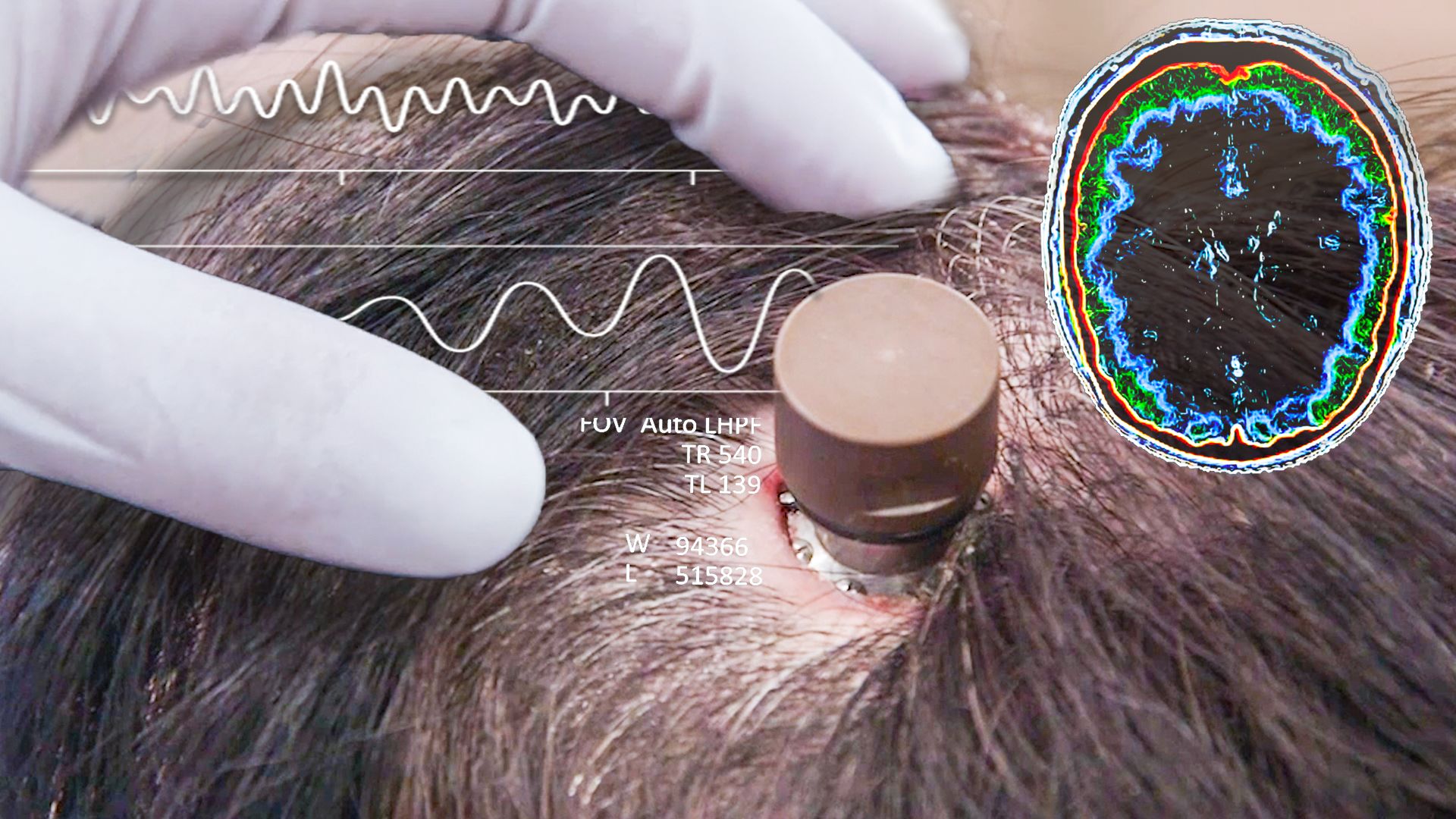
In response to these complications, Neuralink has made several adjustments, including modifying the recording algorithm and enhancing the user interface. These efforts aim to refine how the signals are translated into cursor movements, thereby maintaining the functionality of the BCI despite the setbacks.
The Patient’s Perspective: A Glimpse into a Transformed Life
Despite the technical difficulties, Arbaugh has continued to use the Link extensively, engaging with the system for up to 10 hours on weekends. He describes the experience as a “luxury overload,” highlighting how the technology has reconnected him with the world. This personal testament underlines the transformative potential of BCIs, even in the face of operational challenges.
Neuralink’s Journey: Pioneering Human-Machine Interaction
While Neuralink has made significant strides in BCI technology, the path to commercialization is filled with rigorous safety and efficacy testing. The company must secure approval from the U.S. Food and Drug Administration (FDA) before the Link can be made widely available to those who might benefit from it.
As Neuralink navigates these early hurdles, the scientific community and potential users watch closely. The success of this technology could redefine the boundaries of human-machine interaction and provide unprecedented autonomy to individuals with severe physical limitations.
In this journey of innovation and discovery, Neuralink remains a key player in the quest to merge the human brain with digital technology, promising a future where thoughts might directly interact with the external world. The lessons learned from these early challenges will undoubtedly shape the evolution of BCI technology, bringing us closer to a world where such devices are safe, effective, and accessible to those in need.