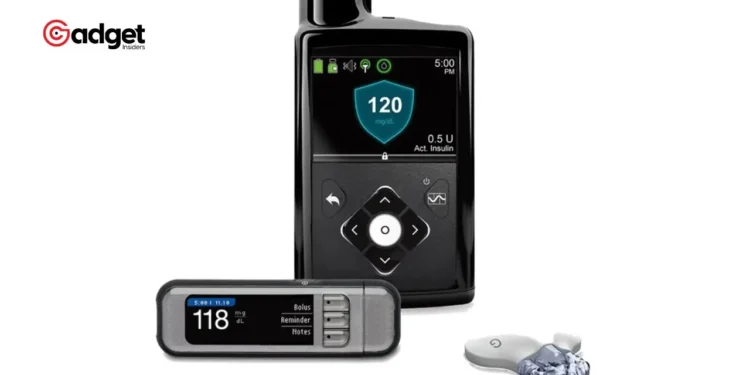
In a significant development that has shaken the diabetic community and medical device industry, the U.S. Food and Drug Administration (FDA) has initiated a Class I recall of the t:connect mobile app. This app, developed by Tandem Diabetes Care, plays a crucial role in managing the t:slim X2 insulin pump, a device that is integral to the daily lives of many people with diabetes.

The Fault in Our Apps: Uncovering the Bug
The t:connect app, heralded as the first smartphone application capable of programming insulin doses approved by the FDA, has been found to contain a severe software bug. This flaw has resulted in repeated crashing and relaunching cycles due to excessive Bluetooth communication, inadvertently leading to rapid battery depletion in the t:slim X2 insulin pump. Such unexpected shutdowns of the insulin pump can be particularly dangerous, potentially causing the under-delivery of insulin to users.
The FDA has issued a statement following the Class I recall for an IOS app used in conjunction with insulin pumps after 224 injuries were reported. https://t.co/GPXAoPW3gc
— NBC News (@NBCNews) May 9, 2024
Consequences of a Compromised Control
When the insulin pump fails to deliver the necessary doses, users are at a heightened risk of hyperglycemia and diabetic ketoacidosis—a serious condition where the body cannot convert sugar into energy due to insufficient insulin. This can lead to life-threatening complications if not managed promptly. Despite the severity of the issue, no deaths have been reported; however, the FDA has received 224 injury reports as of mid-April, highlighting the significant impact of this software malfunction.
Immediate Actions and Preventive Measures
In response to this critical issue, Tandem Diabetes Care issued an emergency notice to all affected customers in March, advising them to update their application, monitor their pump’s battery life closely, and carry backup insulin supplies. This proactive communication aims to mitigate the risks associated with the app malfunction and ensure that users remain informed and prepared.
FDA Recall: Vital Reminder for Insulin Pump Users
The FDA’s recall serves as a crucial reminder for all insulin pump users to stay vigilant. Historically, malfunctioning insulin pumps have been linked to multiple fatalities across various brands. Users are encouraged to regularly check their devices and not to disregard manufacturer alerts or FDA notices regarding such critical updates.
Navigating Through Technological Advances
This incident underscores the complex interplay between technology and healthcare, where innovative solutions like smartphone-controlled insulin pumps can introduce new challenges and risks. It highlights the necessity for continuous monitoring and quick responsiveness from both manufacturers and health authorities to safeguard public health.
As we continue to navigate the integration of technology in healthcare, it remains imperative for users, healthcare providers, and manufacturers to maintain open lines of communication and prioritize safety in the management of chronic conditions like diabetes.