This article dives into the FDA’s warnings, the dangers posed by these faux devices, and the importance of adhering to medically approved health monitors. In a world where technology seamlessly integrates into every aspect of our lives, the allure of convenience often overshadows the critical eye for legitimacy and safety. Recently, the US Food and Drug Administration (FDA) has sounded the alarm on a particularly concerning trend: the rise of counterfeit blood sugar monitoring devices, masquerading as smartwatches and wearable rings.
These devices, often found on popular online marketplaces, promise revolutionary non-invasive glucose monitoring without the backing of scientific approval or clinical evidence.
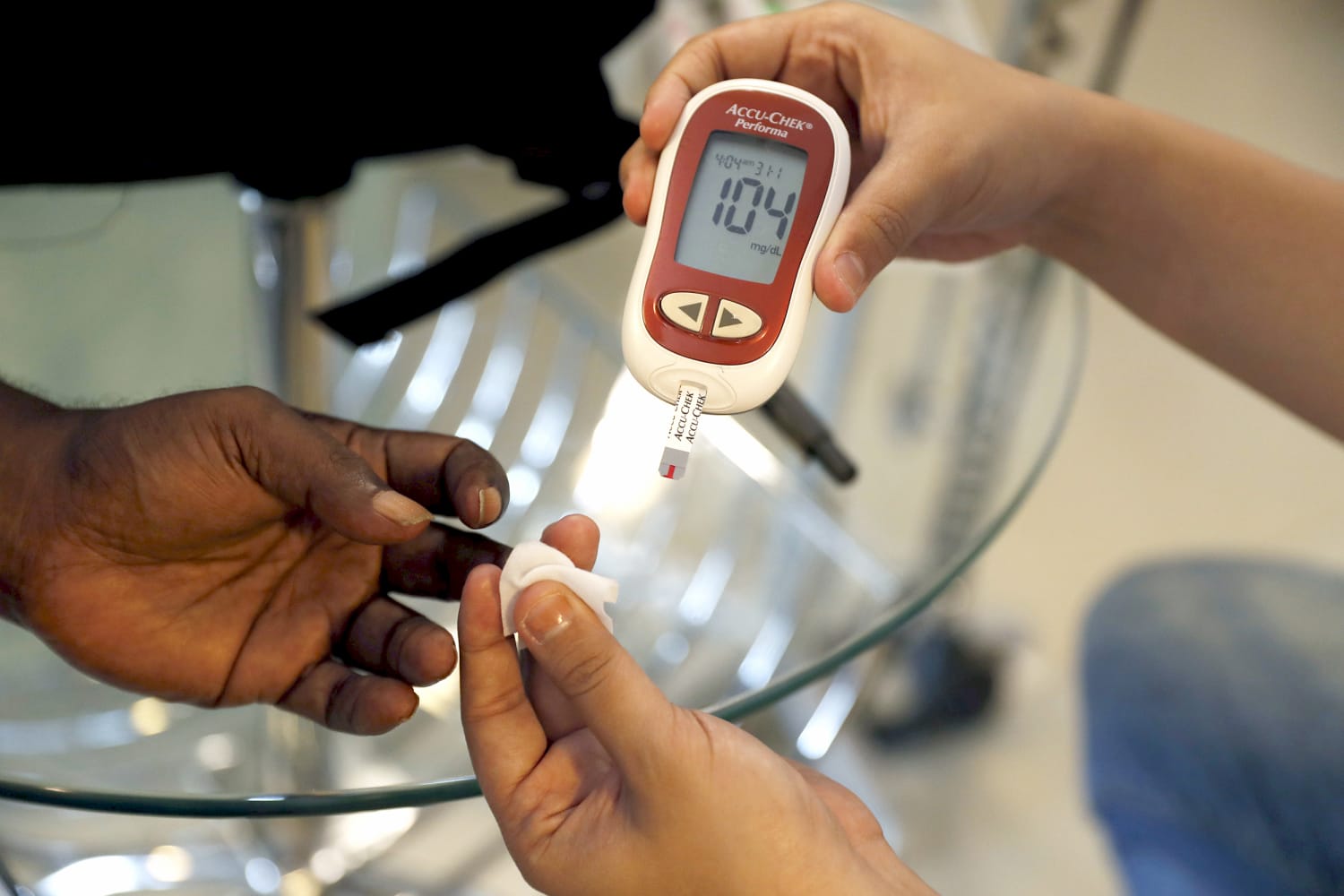
The FDA’s Warning: A Call for Vigilance
The FDA’s recent announcement serves as a stark reminder of the proliferation of unapproved medical devices on the market. “Do not buy or use smartwatches or smart rings that claim to measure blood glucose levels,” the FDA starkly warned, emphasizing that none of these devices have received the necessary authorization, clearance, or approval from the agency.
This warning sheds light on the growing concern over devices that offer health monitoring features without any scientific validation or regulatory approval.
The Allure of Counterfeit Devices
A simple search for “blood sugar ring” yields numerous results, showcasing cheap metal rings and smartwatches that claim to monitor glucose levels using “magnetic fields” or other baseless methods. These products not only fail to deliver on their promises but also mislead consumers with claims of magical health benefits, ranging from blood sugar control to anti-snoring solutions.
The danger lies not just in their ineffectiveness but in the false sense of security they provide to users who may rely on them for critical health monitoring.
People with diabetes don’t need to monitor their blood sugar bc FAKE NEWS !!!!#GQPTraitorsToDemocracy https://t.co/wPUbEsZHvy pic.twitter.com/onlkrp0B7t
— X (@AlmostFamous412) November 7, 2021
The Reality Behind Non-Invasive Claims
The market is awash with websites and online stores promoting these so-called “non-invasive glucose monitoring smartwatches.” These platforms often mimic legitimate e-commerce sites, complete with convincing reviews and testimonials.
However, as the FDA points out, these devices do not perform any form of direct blood glucose testing. Instead, they make unfounded claims about their capabilities, exploiting the demand for non-invasive health-tracking solutions.
Seeking Legitimate Alternatives
While the promise of needle-free blood glucose monitoring is appealing, the reality is that such technology is still under development and has yet to receive FDA approval. Major tech companies like Apple and Samsung are among those researching potential solutions, but until these innovations are scientifically validated and approved, they remain out of reach for consumers.
For those in need of reliable glucose monitoring, the FDA advises consulting with healthcare professionals to explore FDA-authorized devices that offer accurate and safe health tracking.

The Bottom Line
In an era where health and technology intersect more than ever, the importance of vigilance and informed decision-making cannot be overstated. The FDA’s warning is a crucial reminder to consumers to prioritize safety and efficacy over convenience and novelty.
By opting for FDA-approved medical devices and consulting with healthcare providers, individuals can ensure that they are not only monitoring their health effectively but also protecting themselves from the dangers of counterfeit technology.